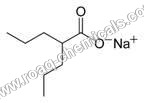
Sodium Valproate IP
Product Details:
- Place of Origin India
- Storage Room Temperature
- Purity 99%
- Appearance White crystalline powder
- Application Pharmaceutical Industry
- Form Powder
- Click to view more
X
Sodium Valproate IP Price And Quantity
- 25 Kilograms
Sodium Valproate IP Product Specifications
- Room Temperature
- 99%
- White crystalline powder
- Pharmaceutical Industry
- India
- Powder
Sodium Valproate IP Trade Information
- 25000 Kilograms Per Month
- 7 Days
Product Description
With rich domain expertise, we have been consistently engaged in providing premium grade Sodium Valproate IP. This valproate is formulated using supreme grade chemical compounds and cutting edge methodology by deft professionals. Offered valproate is extensively demanded in pharmaceutical industry for making medicine used in the treatment of neurological disorder with epilepsy. This Sodium Valproate IP can be purchased from us at remarkable prices.
Quality Key Points:
- Non toxic
- Stabilize electrical activity
- Suitable for both adult and children
- High effectiveness
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email