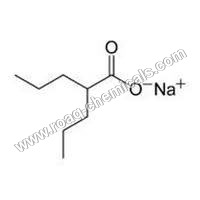
Sodium Valproate BP
1300 INR/Kilograms
Product Details:
- Storage Room Temperature
- Place of Origin India
- Molecular Weight 166 gm/mol Grams (g)
- Other Names Sodium Salt of Valproic acid, Sodium 2- propyl pentanoate
- Type Other
- Usage Anti-epileptic
- Purity 99%
- Click to view more
X
Sodium Valproate BP Price And Quantity
- 25 , , Kilograms
- 1300 INR/Kilograms
Sodium Valproate BP Product Specifications
- India
- 99%
- White
- Room Temperature
- Pharmaceutical Industry
- Other
- White crystalline powder
- Anti-epileptic
- 166 gm/mol Grams (g)
- Sodium Salt of Valproic acid, Sodium 2- propyl pentanoate
- No Smell
- Powder
Sodium Valproate BP Trade Information
- 25000 , , Kilograms Per Month
- 7 Days
- Yes
- Free samples are available
- 50 lt HDPE Drums
- Central America, South America, Middle East, Asia, Africa
- , All India, South India, Central India, North India, East India, Gujarat, Karnataka, West India, Mizoram, Bihar, Odisha, Assam, Dadra and Nagar Haveli, Haryana, Kerala, Lakshadweep, Manipur, Chhattisgarh, Meghalaya, Andhra Pradesh, Chandigarh, Daman and Diu, Goa, Jharkhand, Tamil Nadu, Telangana, Uttar Pradesh, Uttarakhand, West Bengal, Punjab, Delhi, Andaman and Nicobar Islands, Arunachal Pradesh, Madhya Pradesh, Nagaland, Sikkim, Pondicherry, Himachal Pradesh, Jammu and Kashmir, Maharashtra, Rajasthan, Tripura
- GMP, ISO 9001:2015
Product Description
We have carved a niche in this domain by offering top grade Sodium Valproate BP. This valproate is formulated using quality examined chemical compounds by our adroit professionals at our advanced processing unit. Provided valproate is extensively used for the treatment of neurological disorder like epilepsy thus highly demanded in pharmaceutical sector. Our precious customers can buy this Sodium Valproate BP from us at rock bottom prices.
Major Product Points:
- Longer shelf life
- No harmful effects
- Adulteration free
- Accurate composition
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email


 Call Me Free
Call Me Free
