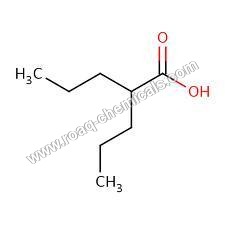
Valproic Acid BP
Product Details:
- Color White
- Place of Origin India
- Storage Instructions Cool & Dry Place
- Form Powder
- Purity 99 %
- Product Type Valproic Acid BP
- Click to view more
X
Valproic Acid BP Price And Quantity
- 25 Kilograms
Valproic Acid BP Product Specifications
- Cool & Dry Place
- 99 %
- India
- White
- Valproic Acid BP
- Powder
Valproic Acid BP Trade Information
- 25000 Kilograms Per Month
- 7 Days
Product Description
Since our inception in the year 1989, we have been engrossed in providing the pristine grade Valproic Acid BP. This acid is formulated using top quality chemical compounds by our dexterous professionals at our advanced production unit. Offered acid is very effective in the treatment of partial, generalized and absence seizures. Additionally, this acid is used for curing migraine headaches. This Valproic Acid BP can be availed from us at nominal prices.
Key Points:
- Longer shelf life
- Non toxic
- Adulteration free
- Safe to use
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email



 Call Me Free
Call Me Free
